“Buildings of this technological era usually deliberately aim at ageless perfection, and they do not incorporate the dimension of time, or the unavoidable and mentally significant processes of aging: this fear of the traces of wear and age is related to our fear of death.”
We try to avoid it: corrosion, wrinkles, or any other signs of aging.
Perhaps as architect Juhani Pallasmaa—author of The Eyes of the Skin—stated, the reason we prefer materials that don’t show age like glass or synthetic plastics is because we don’t want to be reminded of our immortality.
But what if we celebrate the pass of time?
Based on the work of the designer Lex Pott (and as part of the graduate studies I’m taking—a Master in Design through New Materials) we did a workshop at ELISAVA to decode and experiment with the way corrosion works.
True Colours Panels. Lex Pott. (2010)
Since several people asked me to share the process I’m detailing a basic recipe to create a patina with copper below:
Materials:
Copper foil
1 tablespoon of salt
1/2 glass of water
1/2 glass of ammonia
Plastic bottle with a diffuser
Rectangular plastic container (larger than the copper foil) with a lid
Adhesive tape
Plastic bottle caps
Extra tools: tweezers and gloves.
Process:
With the adhesive tape cover the part of the metal that you do not want to corrode (or you can play with strips of tape to create patterns).
Mix the water with the salt until it dissolves inside the plastic bottle.
Add the ammonia into the plastic container (NOTE: please be careful with the ammonia! Use gloves and ventilate the space, the smell is really strong.)
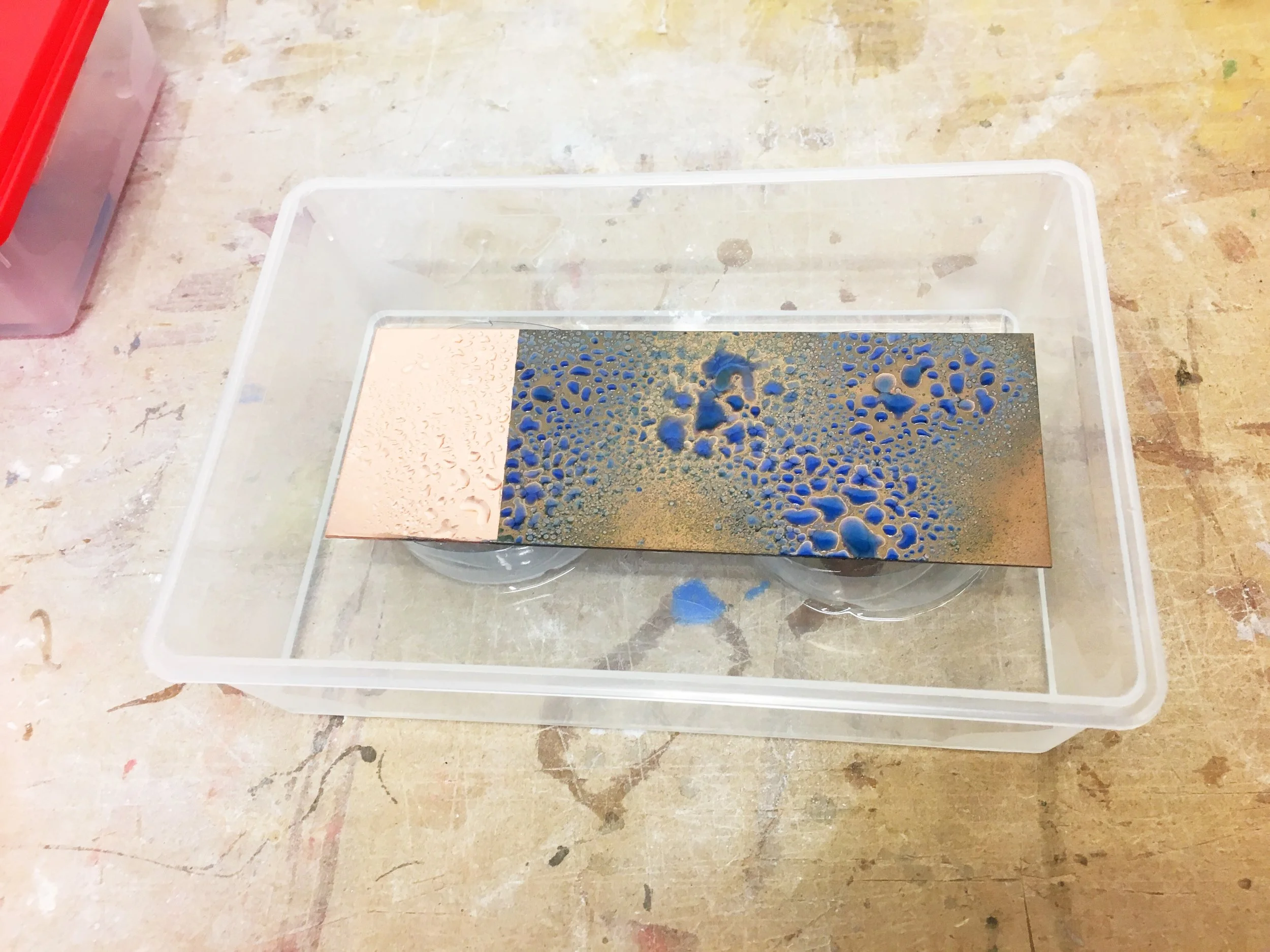
Put the plastic caps in the plastic container for support in the corners and place the copper foil above (ammonia should not touch the metal).
Spray the metal with the water and salt solution.
Close the container so that the gases of the ammonia do not escape.
Now you have to leave the container and wait. The more time you leave it the more intense the color will be. When you are happy with the results remove the lid and take out the metal foil carefully, (so it does not fall into the ammonia or the color will wash out—tweezers can be helpful) and clean the piece.
P.S.: the color comes off when you rub it so add a layer of lacquer if you want to preserve it.
From here you can try different variations; there are a lot of formulas available online for you to test with materials such as brass, bronze, and copper like these from ScienceCompany.
Result of the experiment with the previous recipe and process in copper after 2 hours aprox.
All matter exists in the continuum of time; the patina of wear adds the enriching experience of time to the materials that surround us. This is just one (quite accelerated) example of how natural materials can express their age, as well as the narrative of their origins and their history of human use.
As much as we try to hide it, there lies potential to tell a story...
References
(1) Pallasmaa, J. (1996) The Eyes of The Skin. Great Britain: Wiley-Academy.
(2) Pott, L. Lex Pott: Portfolio. Retrieved October 30, 2018 from: http://www.lexpott.nl/